About service
To address the challenges clients will face in clinical project management in parallel with rapidly changing clinical markets, C&R Research provides a variety of Clinical e-Solution services with optimized e-Solution. C&R Research’s in-house program LeadTrial, coupled with Medidata’s personalized building service, helps clients undertake their clinical project management in an efficient and flexible manner.
LeadTrial (EDC, CTMS)
Medidata (Balance RTSM, Rave, RaveX, etc.)
CRS cube (CDMS, IWRS)
Imtrial
An easy, rapid LeadTrial EDC platforms developed by C&R Research
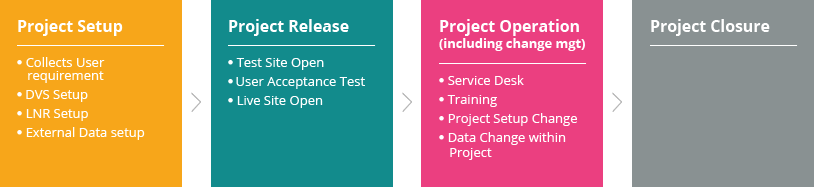
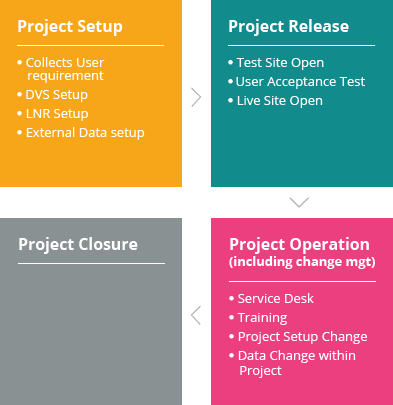
LeadTrial EDC (electronic data capture) platform developed by C&R Research’s 20 years of clinical experience and know-how provides an intuitive user interface and operating method by placing its top priority on business environments and convenience of users (sponsor, sites, monitors). Among other things, Centralized Monitoring assistance function, which can identify multilateral analysis of collected data at a quick glance, is one of LeadTrial’s features differentiating from others.
LeadTrial EDC solution’s efficient and intuitive configuration tool improves easy, rapid case report form (CRF) setting. Programming-free data validation specification (DVS) set-up accelerates Study Start-Upby a maximum of 30 to 50%, reducing time and costs of clients effectively.
Through 21 CFR Part 11 compliant system (US FDA requirement) and GCP-based system validation, C&R strives to provide global e-Solution services for clients.
Global clinical project partner
In an effort to provide its optimized services tailored to global clinical project of clients, C&R Research executed a partnership agreement with Medidata, the world’s No. 1 Clinical e-Solution company and delivers Medidata RAVE, along with other EDC environments customized to the needs of clients.
For more information