[Edaily] C&R Research selected for government task related to standardization of clinical trial data
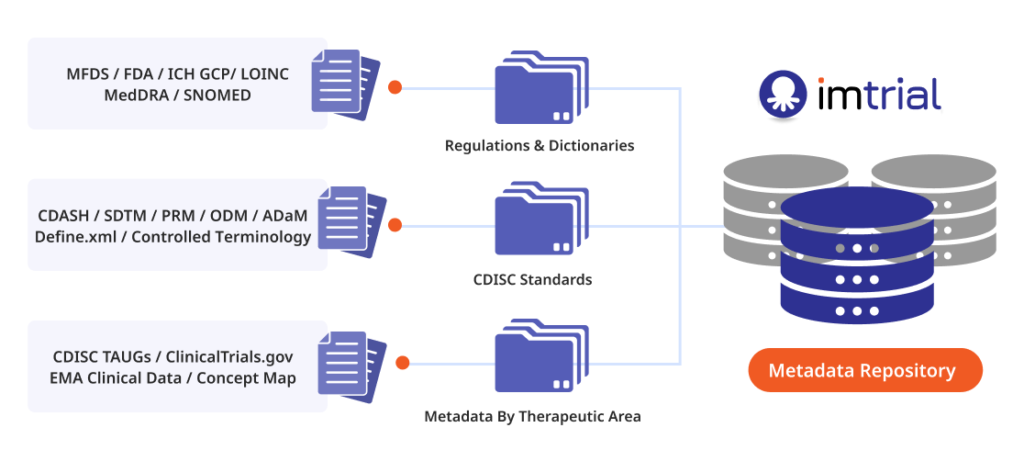
C&R Research (359090) (CEO Moon-Tae Yoon), Korea’s largest clinical trial consignment agency, is carrying out ‘data standardization and application tasks related to clinical trials for each disease’ among the ‘smart clinical trial new technology development research projects’ of the ‘Smart Clinical Trial New Technology Development Research Project Group’. It was announced on the 27th that it had been selected as a joint research and development institution for the project.
This project was carried out by eight industrial, academic, research, and medical institutions, including C&R Research, Kyunghee Medical Center, Kakao Healthcare, and Sungkyunkwan University College of Pharmacy, from the initial planning stage to standardize domestic clinical trial data and prepare innovative plans for clinical research application. An official said that it was achieved through careful consideration together.
