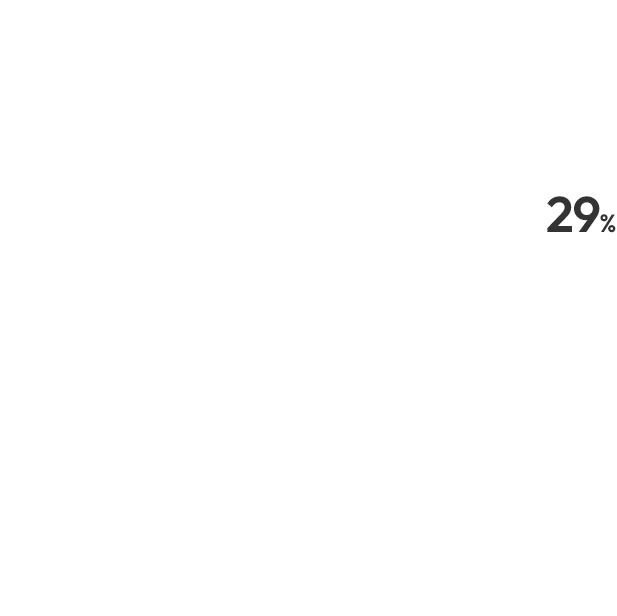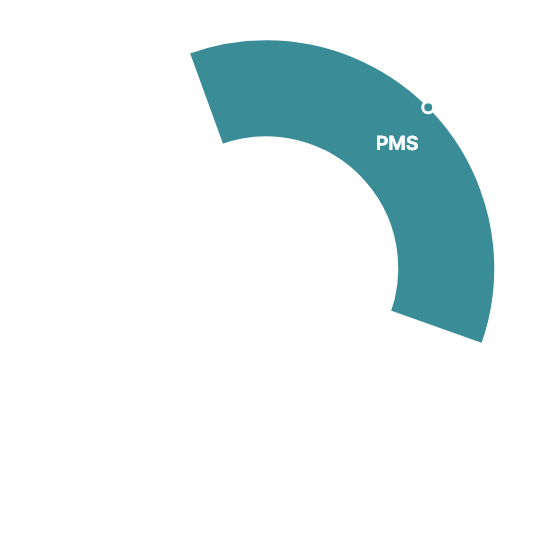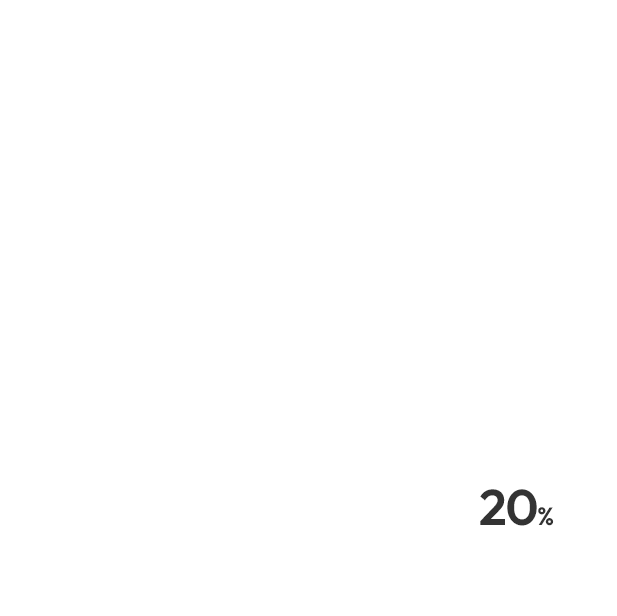
Navigator of Clinical Trials
C&R
Navigator of
Clinical Trials
C&R
Navigator of Clinical Trials
C&R
Navigator of
Clinical Trials
C&R

C&R Research Drives Success
In Clinical Pathways
In Clinical Pathways
C&R Research Drives Success
In Clinical Pathways
In Clinical Pathways

End to End
Service Provider
C&R
-
Since 29
-
Member 500
-
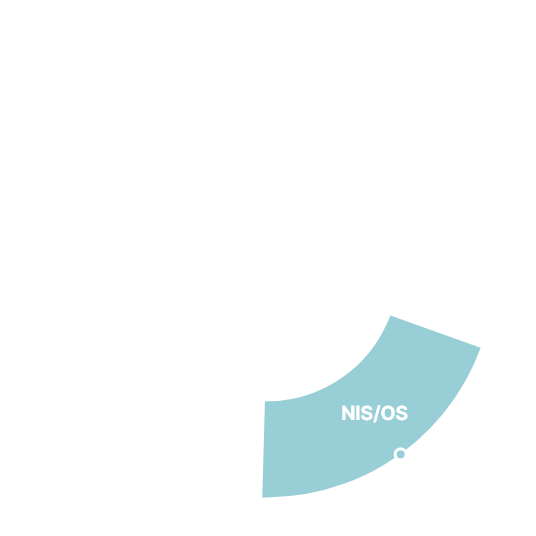
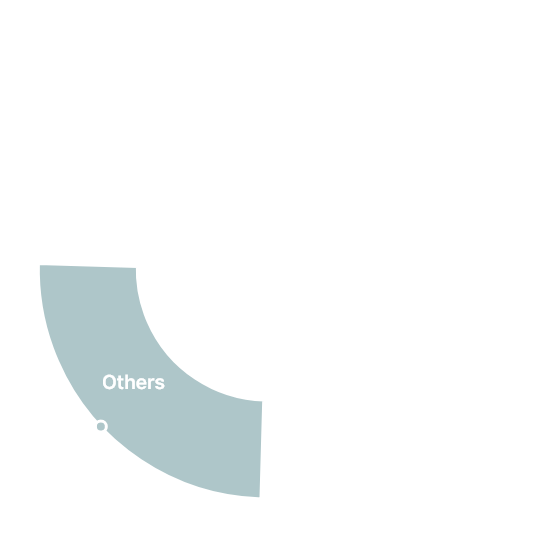
Clinical Trials 1,800
-
Global Clinical Trials 79
Full Scope Service
Pivotal trials
Non-registrational Trials
[2010-2025.4Q]
Prominent Network

Leading the
Future of Clinical Trials
With Integrated Services
Future of Clinical Trials
With Integrated Services
News

Be The Best,
Lead First